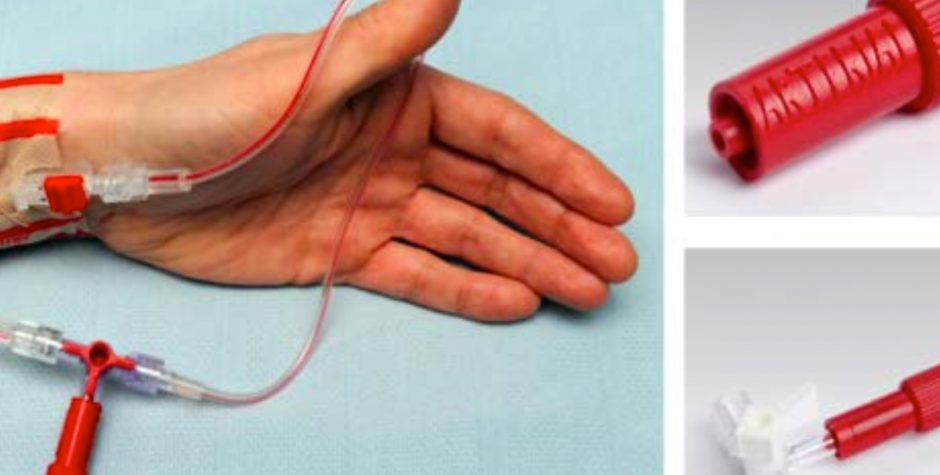
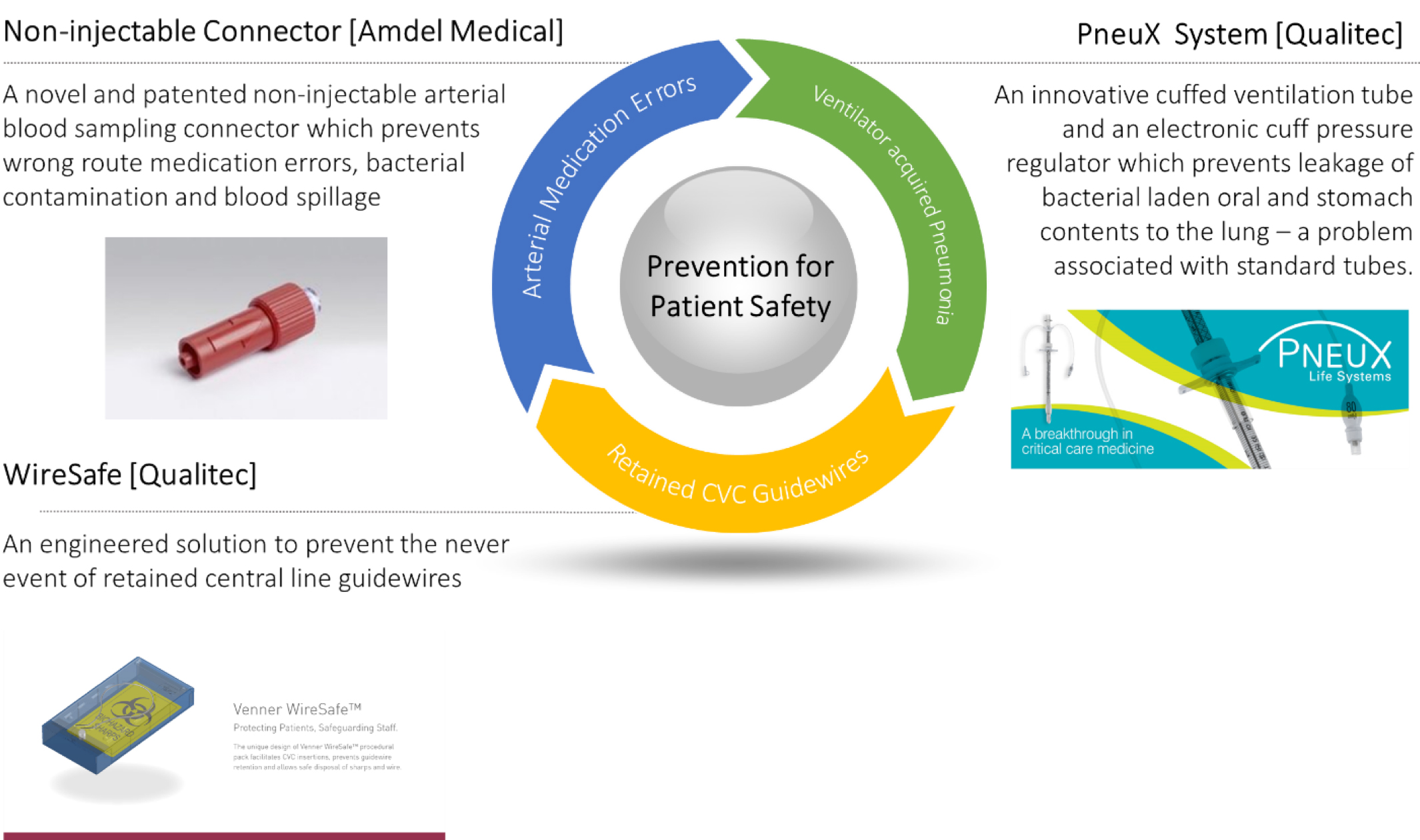
Patient Safety Devices
The CIA team is working closely with innovators who have developed several patient safety devices – PneuX System; Non-Injectable Arterial Connector (NIC); the WireSafe – which are designed for use in critical care and theatres. The three devices have been recognised by the NHS Innovation Accelerator (NIA) programme, with the PneuX System and NIC attracting the NHS England Innovation and Technology tariff from April 2017. This tariff makes the devices free for providers for 2 years.
For more information about the critical care patient safety innovations project please click here
Background Information/Evidence Links
NIC
www.KLIPSuk.com
www.ncbi.nlm.nih.gov/pmc/articles/PMC4472680/
www.eahsn.org
PneuX System
Significant reduction in ventilator-associated pneumonia with the Venner-PneuX System™ in high-risk patients undergoing cardiac surgery: the Low Ventilator-Associated-Pneumonia study. Eur J Cardiothorac Surg. 2015;47(3):e92-6
Luckras 2016, VAP cost effectiveness study, 29th EACTS Annual Meeting
WireSafe
Guidewires Unintentionally Retained During Central Venous Catheterization. J Assoc Vasc Access 2014; 19:29-34
www.england.nhs.uk/patientsafety/never-events/ne-data/
Preventing central venous catheter guidewire retention using a novel safety procedural pack: a pilot clinical simulation randomised controlled trial European Journal of Anaesthesiology 2016; 33(e-supplement 54): 462
Innovation and Technology Tariff
The Innovation and Technology Tariff (ITT) was introduced to incentivise the adoption and spread of transformational innovation in the NHS. It aims to remove the need for multiple local price negotiations and guarantees automatic reimbursement when an approved innovation is used. NHS England has made the NIC and the PneuX System available to provider organisations at zero cost from April 2017 for 2 years.
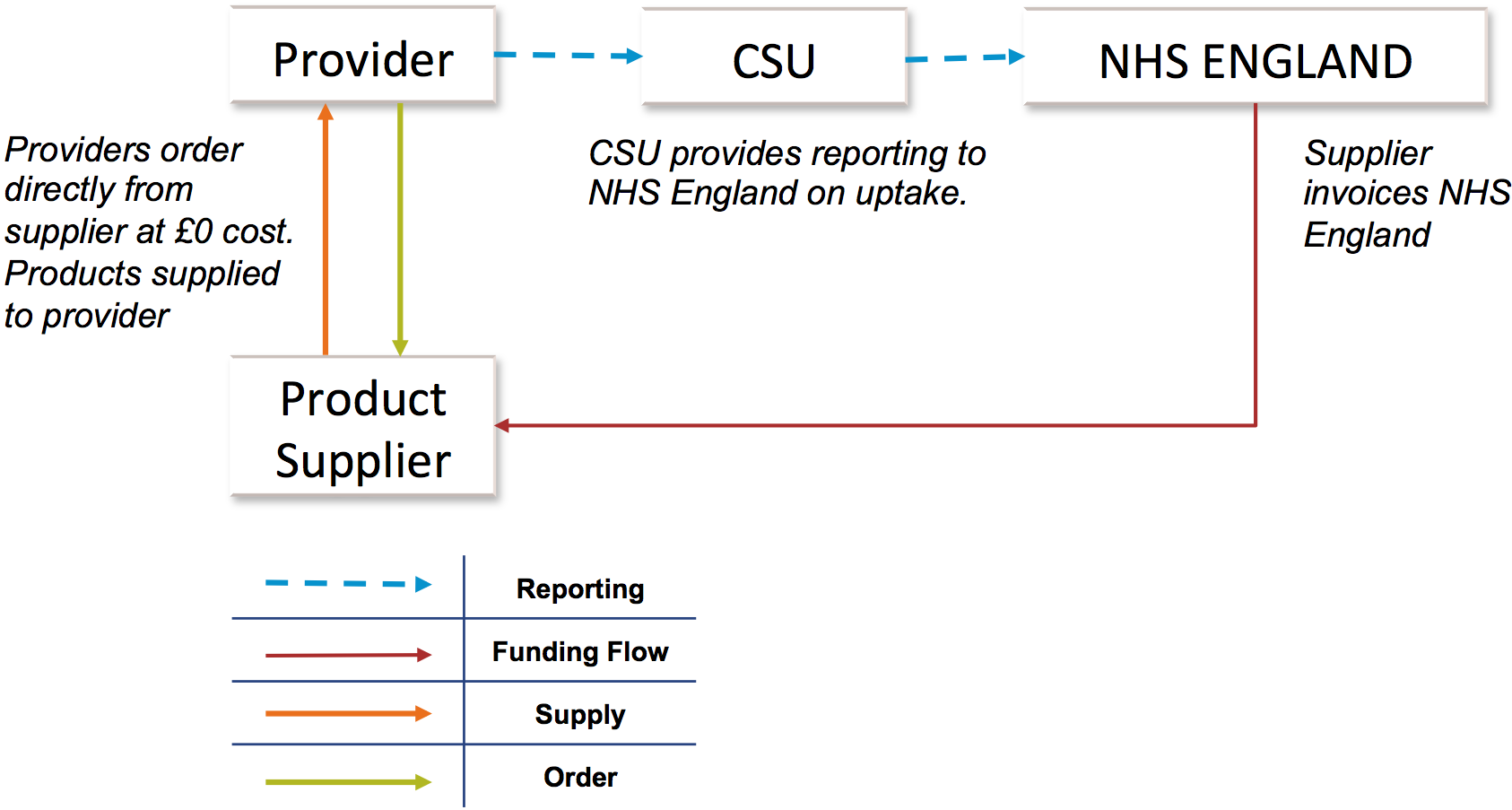
The ITT for the NIC and PneuX System operates under a zero cost model, which has been established to minimise the number of financial transactions and create a more efficient system to administer across the NHS.
Providers will order the NIC or PneuX System direct from the supplier. The supplier will invoice NHS England directly, who in turn will reimburse the supplier.
NHS England has stipulated a minimum dataset for the NIC and the PneuX System in order to monitor usage and impact:
NIC – minimum dataset as requested by NHSE
- Number of patient incidents of bacterial contamination and accidental intraarterial injection prior to the introduction of this innovation
- Number of patient incidents of bacterial contamination and accidental intraarterial injection after the implementation of this innovation
- Number of Non-Injectable Connectors or other approved devices directly used in patient care and a breakdown of usage levels against clinical intervention
PneuX System – minimum dataset as requested by NHSE
- Prevalence of VAP for the previous financial year. This is only required for the first report
- Prevalence of VAP during this period of reporting
- Number of PneuX tubes or other approved VAP prevention devices used on patients ventilated for 24 hours or more
Discussion Topics
- Have you encountered (or do you foresee) any challenges or barriers to implementing these devices?
- Do you feel NHS England’s minimum dataset for the NIC and the PneuX System captures the correct data to monitor impact on patient outcomes? If not, what data do you feel should be collected instead?